- Natural Clay Montmorillonite<Development of Natural Clay Cosmetics>
- QuSome®<Invention of QuSome®><QuSome® Academic Literature>
QuSome® academic literature
Novel Liposomes
Brian C. Keller
Handbook of Cosmetic Science and Technology, 2nd Edition
Edited by A. O. Barel, M. Paye and H. I. Maibach
Introduction
Liposomes are nanosized vesicles (spherical nanocapsules) made of lipids that can only be seen under a microscope. Liposomes are also found in breast milk and widely exist in nature. Liposomes have various known characteristics and are used as effective tools in the field of dermatology. In 1988, Janssen-Cilag launched Peveryl Lipogel, which was the first product to use liposome technology in a local product and was commercialized in Switzerland.
Furthermore, in 10 years, the liposome pharmaceutical formulation ELA-Max was developed and commercialized by Ferndale Laboratories, Inc, and was first announced in the US. Other pharmaceutical companies also utilized liposome encapsulation for the purpose of enhancing functional and local therapeutic effects on the skin.
In Table 1, shows successful dermatological products that have been commercialized using liposome technology.
| Product Name | Drug | Use |
|---|---|---|
| Peveryl Lipogel | Econazole | Mycotic fungal infections |
| Hametum Crème | Hamamelis | Inflammation |
| Heparplus EmGel | Heparin | Anticoagulation |
| L.M.X. | Lidocaine | Dermal anesthesia |
| Miltrex | Miltefosine | Breast cancer tumors with cutaneous metastases |
<Table1>
Since the introduction of Christian Dior's cosmetics Capture® in 1987, liposomes have been known to have several major functions as well as other parts that have not yet been elucidated. The obstacles to using liposomes include cost and formulation difficulties, manufacturing scale issues, stability, and the fact that the value of liposomes is generally misunderstood. However, in recent years, many skincare products have been developed through practical testing.
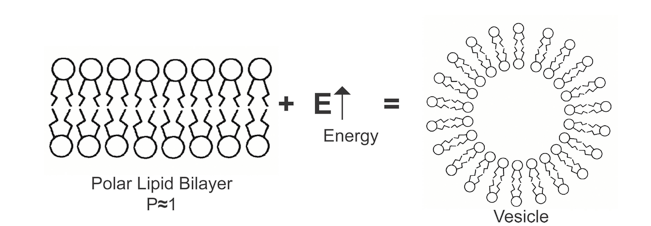
The conventional liposomes described in the previous chapter are made of purified phospholipids, mostly consisting of a mixture of DPPC alone or with cholesterol or other sterols. The function of these amphiphilic lipids is to prepare the liposome to form a bilayer sheet in a polar solvent (usually water). The resulting colloidal aggregate has a hydrophilic (water-soluble) head on the outside and a hydrophobic (water-insoluble) tail on the inside, allowing the formation of a bilayer capsule with the addition of energy from the outside. <Figure 1>
It takes the same amount of energy for you to physically fold one page of this book and insert it into a tube using your hands and fingers as it does to create the capsule of a phospholipid bilayer membrane. This capsule is often thought to be naturally formed, but it is not. The difference between self-formation and natural encapsulation is not clear. The phospholipid bilayer membrane only forms liposomes through self-closure when external energy is applied. The main source of external energy during encapsulation comes from processes such as ultrasonic treatment, high-speed mixing, homogeneous high-pressure treatment, and high-speed cutting fluid treatment.
DPPC=dipalmitoylphosphatidylcholine
<Figure1>
As important derivatives of conventional liposomes, there are nonionic surfactant liposomes and non-phospholipid capsules. Both are generally called synthetic surfactant vesicles (capsules). These vesicles (capsules) are composed of synthetic surfactants with a single or double chain on a nonionic polar head. They form vesicles (capsules) with a bilayer membrane structure in which the hydrophobic tails control the membrane properties.
According to patent documents from the early 1980s, it was found that there were already French and Japanese inventions using this type of technology. Ten years later, this technology was applied to cosmetics. The technology called Niosomes was introduced to Lancôme and other L'Oreal brands in the late 1980s, and other companies followed suit.
Cosmeceuticals and cosmedics, which are medicinal cosmetics containing beauty-activating ingredients, have evolved into products that focus on their formulation and are linked to sensory testing and treatment methods, becoming a topic of conversation. To create a satisfactory finished product using phospholipids, large-scale processing capabilities and high formulation technology are necessary. It is a well-known fact that by doing so, the stability of the resulting liposomes and the encapsulated ingredients can be maintained in good condition.
Colloidal aggregate
<Figure2>
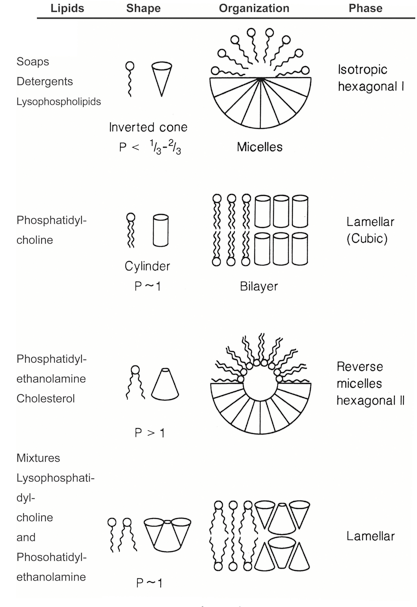
The basic characteristic of lipids is their geometric shape. This metaphysical structure is determined by the packing parameter (molecular shape) of lipid P. The packing parameter (molecular shape) is related to the amount of lipids given and is determined by the size of the polar head and the length of the hydrocarbon chain of the lipid.
According to the experiment, determining the packing parameter P is an effective determinant for determining the shape of the bone material of amphiphilic lipids.
Definition of P:
P = v / al
v:volume of the molecule
a:polar head region
l:length of hydrocarbon chain
Regarding single-chain lipids, detergents, and surfactants, if the polarity head is larger than the non-polarity tail in structure and P is less than or equal to 0.75, a micelle is formed. When the lipid P value is up to 1, it is the most suitable state for forming liposomes. When P is greater than or equal to 1, the polarity head becomes smaller in proportion to the non-polarity chain and inverse micelles are formed. Interesting packing parameters, shapes, and compositions of some lipids used in cosmetic formulations are shown in <Figure2>.
※ micelle::A structure formed by molecules with both hydrophilic (water-loving) and hydrophobic (oil-loving) parts, where the hydrophobic parts are oriented towards the center and the hydrophilic parts are oriented towards the outside, making it easier for the structure to dissolve in oil. For example, a phospholipid bilayer is also a type of micelle.
A Model of Geometric Packing of various amphiphilic lipids into colloidal aggregates.
(Adapted from D.D. Lasic, Liposomes; From Physics to Applications, Elsevier, 1993 pp 51)
In order to form a double membrane layer by assembling lipid head groups and hydrocarbon chains according to the radius of the resulting vesicles (capsules). If the hydrocarbon chain is smaller than the head, the resulting radius increases and a micelle is formed. Conversely, if the hydrocarbon chain is larger than the head, the resulting radius becomes an inverse signal and inverse micelles are formed.
New, naturally occurring and thermodynamically stable liposomes (STS)
Almost all liposomes are known to be thermodynamically unstable. Instead, they can trap stronger energy by using the energy used during their formation. Again, energy is used as heat, ultrasonic processing molding, and homogenization processing. All high energy causes problems such as material aggregation, fusion, settling, and leakage during liposome formation, as it tries to weaken the energy released. However, well-formulated liposomes and their finished products maintain a stable state for a long time and do not scientifically degrade if stored properly. By maintaining the thermodynamic stability of liposomes, many problems can be avoided, which is ideal.
Despite the success and expansion of the application of conventional liposomes, it was necessary to urgently develop a new type of lipid that can form liposomes easily and has a cost-effective advantage to solve some problems.
<Figure3>
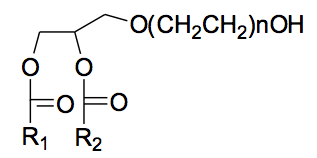
In this chapter, we will describe a new group of lipids that can easily form thermodynamically stable liposomes and have excellent cost-effectiveness. Please refer to <Figure3> for the general structure of this lipid.
This structure is similar to phospholipids, but the fundamental difference is that it is superior in forming thermodynamically stable liposomes. The essential hydrophilic head is a PEG chain with a wide range of 8-45 subunits or 300-5000 Daltons (n = 8-45). PEGylated lipids were bound to the membranes of several liposome compositions (e.g., Stealth®) made from phospholipid liposomes with phosphate head groups. Glycerin, which is mainly composed of a 3-carbon chain, and two hydrocarbon R1 and R2 vary depending on the length (C = 8-25).
※PEG = Polyethylene glycol: Nonionic surfactant
※Dalton = A unit of mass for molecules or atoms. 1Dalton = 1/1000nm
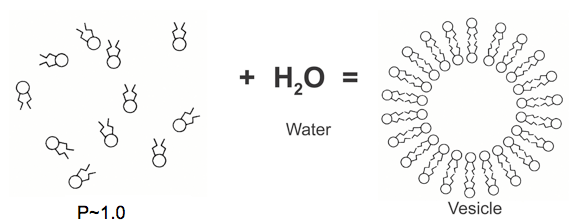
When mixing lipids and aqueous solutions to form liposomes, very little energy is required due to the use of lipid molecules. The lipid molecules mix with water and naturally settle into a low energy state, forming vesicles (capsules). As a result, thermodynamically stable multilayer lipid vesicles (capsules) are formed.
<Figure4> shows the state where lipid forms a capsule (vesicle) at the melting point when mixed with water.
<Figure4>
When using these lipids in cosmetic formulations, it is easy to prepare them in large or small quantities without much effort. These lipids have a low melting point and do not lose their properties even when heated to 75-80°C, demonstrating excellent abilities in various dermatological formulations.
Organic solvents for dissolving lipids are not necessary for formulation; the process only requires mixing in processing equipment to form lipid capsules.
Table2 provides examples of the usage status of lipids in cosmetic formulations.
| Lipid | Melting Point | Spontaneous Liposomes at Melting temperature |
|---|---|---|
| PEG-12 Glyceryl dioleate | Fluid @ 25oC | Yes |
| PEG-12 Glyceryl dimyristate | Fluid @25oC | Yes |
| PEG-23 Glyceryl palmitate | 31.2oC | Yes |
| PEG-12 Glyceryl disterate | 40.0oC | Yes |
| PEG-23 Glyceryl disterate | 39.8oC | Yes |
Table2
Liposomes formed using these lipids have sizes ranging from 750 to 1500 Å, and the thickness of the double layer membrane is approximately 40 Å. Under the microscope, their size appears uniform, but they can occasionally become perfectly spherical, elliptical, or irregular. This is likely due to the purity and homogeneity of the lipids used.
※ Å = Angstrom: a unit of measurement for very small lengths, typically used for molecules and atoms. 1Å = 10-10m = 0.1nm

<Figure5>
In a cosmetic lab, the ideal way to confirm the presence of liposomes in a formulation is through a polarized light microscope. <Figure5> shows pictures of some multilayer liposomes formed from PEG-12GDO and cholesterol. They appear as perfect white spheres with a delineated cross in the center. These remained stable during a 90-day test at very active temperatures of 40 and 50 degrees, and throughout freeze-thaw cycles. During the formulation of cosmetics, these liposomes have a higher level of ionic surfactants and resistance across a wider pH range of 2.5 to 9.18 compared to traditional liposomes. Moreover, PEG-12 GDO and PEG-12 GDM act as excellent solvents, working on hard-to-dissolve components including cholesterol.
Due to their thermodynamically stable state, liposomes do not dissolve, bind, or become unstable to achieve a minimum energy state.
During cosmetic formulation, by using non-phospholipids, it became possible to create products that are not affected by microbial growth due to the lack of essential phosphates as nutrients for microorganisms. The absence of this type of head group prevents the oxidation of the amine in the polar head of phospholipids that causes a fishy odor, bringing overall stability.
The toxicity of phospholipids has been thoroughly reviewed and is essentially non-toxic. A large-scale test on lipid toxicity, as shown in <Table2>, was recently conducted and these were concluded to be non-imitative and non-toxic. These lipids are approved for use in many skincare products.
Practicality
Cosmetics incorporating traditional liposomes are now seen everywhere. However, sometimes the practical significance of liposomes takes a backseat. The existence of liposomes is often advertised on labels or prioritized for marketing purposes. The structure of microscopic capsules riding on the good-looking vehicle of cosmetics is trapped in a situation of bringing consumer effects beyond the limits of cosmetic labels. The content of liposome ingredients related to important treatments should be displayed to ensure effective achievement.
Capsule Encapsulation Efficiency
The advantages of liposomes used in dermatological products include improved solubilization of ingredients, microencapsulation to stabilize cosmetic ingredients, prolonging the time ingredients stay on the skin, sustained release of ingredients for continuous effect, and providing lipid molecules to the skin to improve barrier function and hydration.
When considering the construction of a cosmetic formulation, techniques for mixing liquids, solids, oils, water, crystals, cellulose derivatives, and pastes are needed. As mentioned at the beginning of this chapter, while the therapeutic value of general products is coming into focus, it is a mistake to overlook the touch, feel, and smell of the product. In any case, a lot of time is spent considering the composition of each ingredient.
The concentration of beauty active ingredients is low, and the general usage level is in the range of 1-5%. Moreover, most formulas contain one or more ingredients that bring effects to the skin. To achieve the liposomal encapsulation of the desired active ingredients, you need to understand the physicochemical properties of the materials. Lipophilic components are on the upper side of the capsule and penetrate into the acyl group (fatty acid), while hydrophilic components are in the lower layer of the capsule.
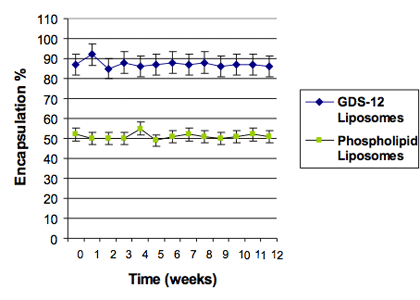
<Figure6>
The type of lipid (encapsulated ingredient, charged lipid, and active ingredient) contributes to improving the efficiency of active ingredient encapsulation. According to experiments, the easiest way to start formulating and maximizing encapsulation is to set the ratio of lipids to ingredients at a 2:1 concentration. For hydrophilic ingredients, a higher concentration ratio may be needed.
In a comparative study of two liposome formulations at equal molar concentrations of lipids, the PEG lipids forming STS liposomes showed greater encapsulation efficiency than traditional liposomes.<Figure6>
※Acyl chain = Lipophilic tail part of a lipid molecule.
※Molar concentration = One way to express the concentration of a solution. It is expressed as the amount of solute in a unit volume of solution.
Penetration from Skin
Even cosmetics use delivery technologies, the main reason being their high skin penetration ability. Delivering active ingredients to the layers beneath the stratum corneum is desirable for many beauty purposes, such as improving hydrophilicity, barrier function, removing reactive oxygen for anti-aging, strengthening the epidermis and dermis with nutrients and vitamins (vitamin A and similar substances), delivering tocopherol and vitamin C, and other known skin active ingredients.
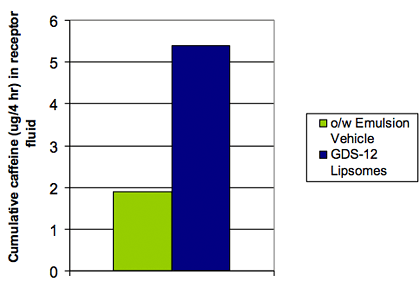
<Figure7>
The technology of encapsulating liposomes for skin penetration in cosmetics and pharmaceuticals had progressed remarkably by the mid-1990s. Many studies have discussed the capabilities of skin penetration and active ingredient encapsulation. This has become a very significant benefit of liposome encapsulation. The use of liposomes has been found to not only accelerate skin penetration of active ingredients but also enhance their "therapeutic effect."
Skin penetration studies using STS liposome-encapsulated caffeine were conducted using deceased human skin.
The situation of continuously flowing and diffusing into cells produced interesting results. The caffeine encapsulated in liposomes made from PEG-12 GDS was compared to caffeine C-14 in the same state, not encapsulated, included in water and oil emulsifiers. 8hours later, further caffeine penetrated the skin and diffused into the receptor solution.<Figure7>
Application of Liposome Technology to Cosmetics
Traditional liposomes are expected to continue to yield results in the fields of therapy and pharmaceuticals. This utilization is recommended for improving spots and whitening products and vitiligo treatment, etc. Encapsulating ingredients after stabilizing them with liposomes and retinol has resolved the problem that there were limits to the amount of active ingredients used in cosmetics. Furthermore, in medicinal cosmetics, molecules that stimulate hair growth and latent DNA can be sent into the pores. The ease of use, cost, stability, and broad effectiveness of STS liposomes have created the potential to use penetration technology in other fields.
In basic research, naturally occurring and stable (STS) liposomes have been confirmed to be practical penetration technology in cosmetic science. This lipid is mainly PEG-12 GDS and PEG-12 GDO, and this technology is used in products for treating acne, dry skin, inflammation, whitening, and aging wrinkles. In laboratory tests, STS liposomes have been proven to be superior to currently available delivery materials in terms of solubilization of ingredients, stability of ingredients, and encapsulation. STS liposomes are also effective options for delivering peptides and their growth factors, hydrolyzed wheat protein, glycolic acid, retinol, co-factors, and co-enzymes.
Conclusion
Since the late 1980s, conventional phospholipids and the vesicle (capsule) delivery system of local formulations, which were restricted by patents, have been made usable for cosmetics due to the emergence of non-phospholipids. Over the past 20 years, we've heard of many successful examples of liposome products. Continued utilization of liposomes and understanding their true value will draw attention to the operating system of cosmetic formulations.
The ideal cosmetic formulation contains an appropriate concentration of active ingredients and is included in creams, gels, lotions, and serums that look good, smell nice, and are easy to use. For manufacturing, it should be something that doesn't require laboratory formulation or large-scale manufacturing processes, or expensive equipment. The new delivery system has significantly contributed to implementing this definition. Currently, there are no obstacles to utilizing liposomes, and they have become an essential tool in dermatological formulations.

